Abstract
Background: IMDs including mucopolysaccharidosis type IH (MPS1/Hurler Syndrome), metachromatic leukodystrophy (MLD), globoid cell leukodystrophy (GLD) and cerebral adrenoleukodystrophy (cALD) are progressive, fatal diseases affecting the central nervous system which are treatable through allogeneic hematopoietic stem cell transplantation (HSCT). CB, in the absence of a matched donor, is the preferred source of stem cells as it is rapidly available and allows greater flexibility in allele matching. As a result of low cell doses, CB transplants in IMD are associated with prolonged periods of neutropenia and reported graft failure rates in up to ~20% (Lum et al 2017 Bone Marrow Transplant 52:846-53; Mallhi et al 2017 BBMT 23:119-25). MGTA-456 is a first-in-class cell therapy produced from a single CB unit using an aryl hydrocarbon receptor antagonist in a 15-day expansion culture of CD34+ cells. In previous phase 1/2 studies, 24 adult and 3 pediatric patients with hematologic malignancies treated with myeloablative conditioning (MAC) and MGTA-456 demonstrated a median 324-fold expansion of CD34+ cells, all patients engrafted, and the time to neutrophil recovery was significantly reduced by a median of 9 days compared to historical controls (Wagner et al 2016 Cell Stem Cell 18:144-55; Wagner et al 2017 Blood 130 supp:662 abstr). Furthermore, higher CD34+ dose has been correlated with improved engraftment and outcomes in IMD transplant patients (Prasad et al 2008 Blood 112:2979-89). Based on these promising data, we postulate the increased CD34+ dose provided by MGTA-456 would reduce the length of neutropenia and risk of graft failure in IMD patients.
Patients and Methods: A Phase 2, open-label trial (NCT03406962) initiated in Feb 2018 is enrolling up to ~12 patients <16 yo with a diagnosis of MPS1, cALD, MLD, and GLD lacking a non-carrier matched related donor. Eligible CB units were matched at ≥ 6 of 8 HLA loci (A, B, C and DRB1) using allele-based typing. The reduced toxicity MAC regimen consists of anti-thymocyte globulin (days -9 to -6) followed by fludarabine (40 mg/m2 days -5 to -2) and busulfan (total exposure 21,000 to 22,000 μM/min/L-1 days -5 to -2). Immunoprophylaxis consists of cyclosporin and methylprednisolone.
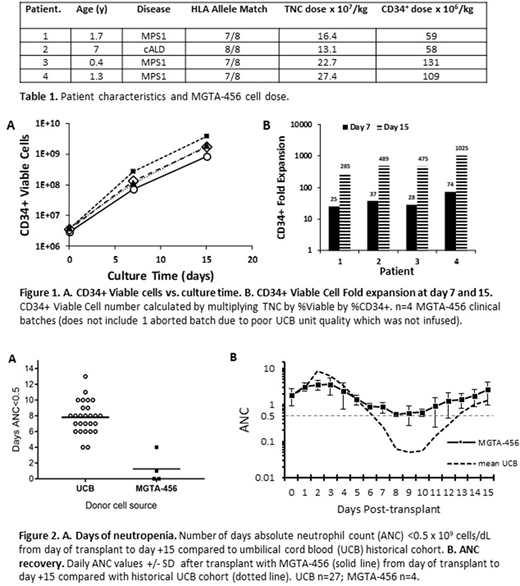
Results: Four patients have been treated thus far (Table 1). The MGTA-456 process provided a marked expansion of CD34+ cells (median 482-fold, Figure 1) with a median infused CD34+ cell dose of 84 x 106 cells/kg and median total nucleated cell (TNC) dose from the expanded fraction of 19.6 x 107 (Table 1). TNC dosing was capped at 27.0 x 107 cells/kg per protocol. The only infusion-related reaction noted was grade 3 nausea in 1 patient. Two patients had no days of neutropenia and 2 patients had 1 and 4 days respectively (mean 1.25 days) in contrast to a mean of 7.8 days for a historical cohort of 27 IMD patients undergoing CB transplantation at the same institution with identical conditioning (Figure 2). Myeloid chimerism (CD33+/66+) achieved ≥98% donor by day +14 in all patients. For the 3 patients with data at time of reporting, days to discharge after transplant were 17, 12 and 18. No patients experienced acute or chronic GVHD. One patient developed autoimmune cytopenia (not related to MGTA-456) which is a known complication reported in 20-56% of IMD patients undergoing HSCT that resulted in death at day +143 (Page et al 2008 BBMT 14:1108-17; Khalil et al 2014 Sci World J 581657). Long-term disease specific outcome measures including enzymatic activity are being collected and will be reported over time.
Conclusions: Preliminary results of transplantation in IMD patients with MGTA-456, containing highly expanded CD34+ cell doses, demonstrated early and robust engraftment in all patients with marked reduction in days of neutropenia (to 0-4 days or mean 1.25 days) in comparison to a historical cohort. MGTA-456 was well tolerated with one infusion-related event of grade 3 nausea. These data, in combination with the previous 27 hematologic malignancy patients treated, suggest that MGTA-456 substantially enhances the engraftment potential of CB. Based on these promising data, MGTA-456 has potential to improve transplant-related outcomes in patients undergoing HSCT and increasing the availability of well-matched CB units that may have been previously excluded due to inadequate CD34+ dose.
Orchard:Magenta Therapeutics: Research Funding. Raffel:Magenta Therapeutics: Employment, Equity Ownership. Condon:Magenta Therapeutics: Employment, Equity Ownership. Monaghan:Magenta Therapeutics: Employment, Equity Ownership. Vernet:Magenta Therapeutics: Employment. Sheirr:Magenta Therapeutics: Employment, Equity Ownership. Braun:Magenta Therapeutics: Research Funding. Shanley:Magenta Therapeutics: Research Funding. Lund:Magenta Therapeutics: Research Funding. Boitano:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Cooke:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Davis:Magenta Therapeutics: Employment, Equity Ownership. Wagner:Novartis: Research Funding; Magenta Therapeutics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal